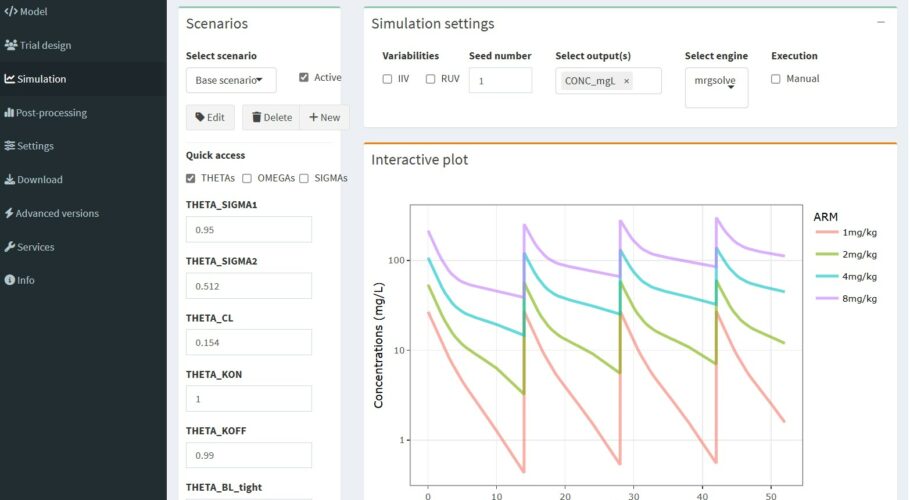
This model is an adaptation of the second-generation minimal PBPK model for monoclonal antibodies initially described by Cao et al. 2013 and later refined and applied to interspecies-scaling by Zhao et al. 2015. Physiological parameters like lymph or plasma flow and volumes are scaled by body weight, to allow simple scaling between species on the basis of body weight. Binding of the antibody to it’s target occurs in the plasma compartment and is handled by the full target-mediated drug disposition (TMDD) approach with the following parameters:
– BL_target: Baseline target concentration (nM)
– KD_target: Equilibrium dissociation constant (nM)
– KOFF_target: Rate constant of dissociation of the antibody-target complex (1/day)
– KDEG_target: Degradation rate of the free target (1/day)
– KINT_target_AB: Internalization or degradation rate of the antibody-target complex (1/day)
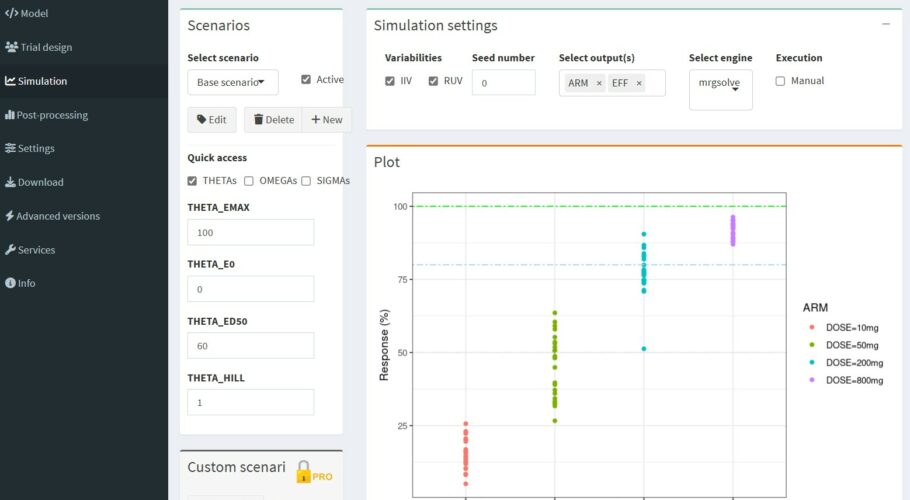
This is a straight-forward example of a sigmoidal dose-response model to illustrate how you can easily simulate non-longitudinal models with e-Campsis. In the present case we use a dosing compartment where the amount per arm is injected as a bolus. Since we have no time-dependency, we only need to specify a single observation time point.
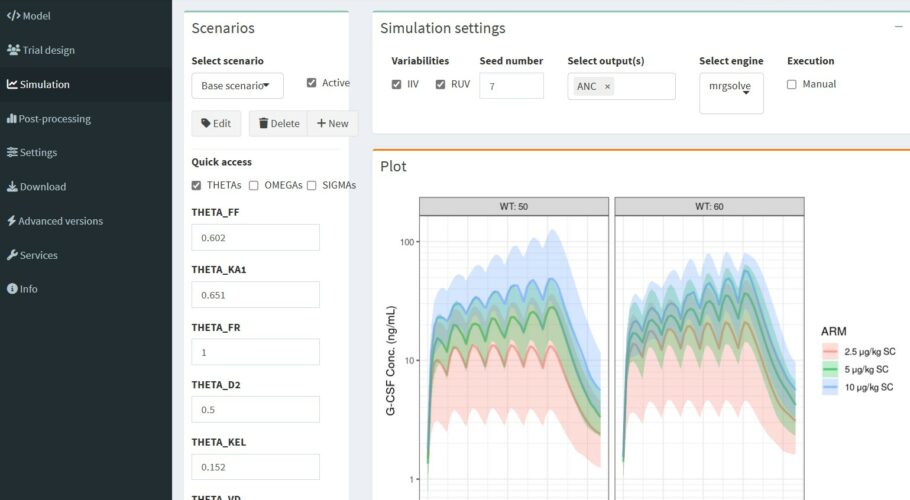
Filgrastim is a recombinant human granulocyte colony stimulating factor (G-CSF) that stimulates production of neutrophils. The model depicted the decaying trend in Cmax values with repeated doses and an increase in absolute neutrophil count (ANC) consistently with an increase in the G-CSF receptor pool. Simulated time courses of the total clearance exhibited an increasing pattern. The increase in filgrastim clearance on multiple dosing was attributed to the increased neutrophil count in the bone marrow and blood paralleled by an increase in the total G-CSF receptor density.
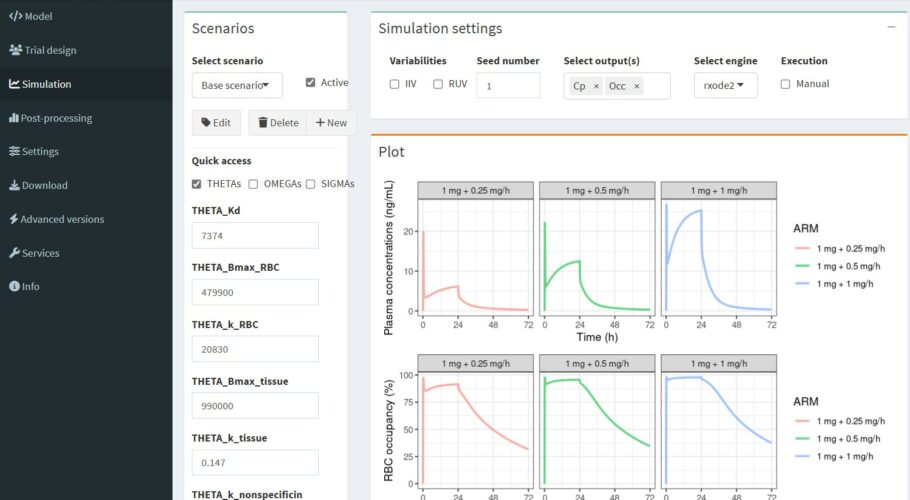
The red blood cell/plasma distribution of draflazine (a nucleoside transport inhibitor which is cardioprotective due to potentiation of receptor mediated effects of adenosine in the ischemic myocardium) was non-linear and characterized as a capacity-limited specific binding to the nucleoside transporter on the red blood cells. Binding to the nucleoside transporter on red blood cells is an important determinant of the pharmacokinetics of draflazine and a high degree of occupancy of the transporter by draflazine is required to inhibit adenosine breakdown ex vivo. It is suggested that the red blood cell nucleoside transporter occupancy may serve as a useful pharmacodynamic endpoint in dose ranging studies with draflazine.
Discover how e-Campsis can enhance your PK/PD
simulations. Start for free or request a demo to explore
the full potential.